[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)
date : 12/16/2025
Tags: Cancer, NCDs, Precision Cancer Medicine
![[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/eyecatch_Policy-Dialogue_Discussion-Points_20251128.jpg)
Health and Global Policy Institute (HGPI) has published the discussion points “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today.”
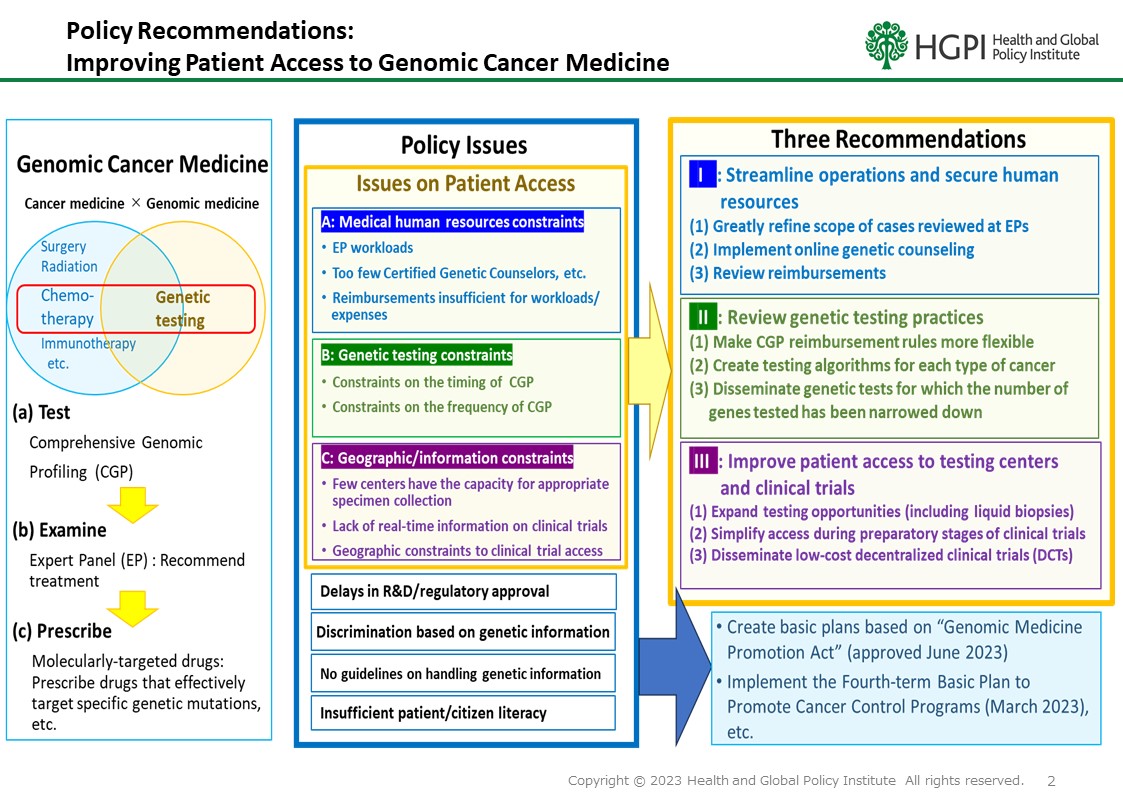
Since comprehensive genomic profiling (CGP) was granted insurance coverage in 2019, people serving in clinical settings have built experience with genomic cancer medicine, and findings from various studies continue to accumulate. In June 2023, Japan enacted the “Act on the Comprehensive and Systematic Promotion of Policies to Ensure that the Public Can Receive High-Quality and Appropriate Genome Medicine with Peace of Mind” or the Genome Medicine Promotion Act, and momentum for the promotion of genomic cancer medicine continues to grow. However, various issues must be addressed before appropriate genomic cancer medicine can be broadly provided to patients.
On June 11, 2025, a joint report titled “Briefing Report on Solid Cancer Treatment Based on Gene Panel Testing Using Next-Generation Sequencers” was presented by the Japanese Society of Medical Oncology (JSMO), the Japanese Cancer Association (JCA), and the Japan Society of Clinical Oncology (JSCO). Guidance provided by these three societies was utilized as an important foundation when CGP was granted insurance coverage in 2019, and the 2025 Briefing Report is another resource that should be used as guidance during the next revision of the medical service fee schedule. Now is precisely the time that it is critical for us to deepen discussions and take concrete action to promote genomic cancer medicine so its benefits can be delivered to patients in a more appropriate manner.
In light of this, HGPI hosted a policy dialogue convening stakeholders from industry, government, academia, and civil society to exchange views on the current landscape, ongoing challenges, and future directions of cancer genomic medicine in Japan.
This discussion points document outlines the concrete actions and future directions that stakeholders across industry, government, academia, and civil society must pursue to ensure that cancer genomic medicine truly translates into tangible benefits for patients.
For details, please see the PDF at the bottom.
Top Research & Recommendations Posts
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Policy Recommendations] Mental Health Project: Recommendations on Three Issues in the Area of Mental Health (July 4, 2025)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Announcement] HGPI Endorses the “Belém Health Action Plan” (November 14, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Policy Recommendations] Recommendations on Strategic Investments in Policies for Brain Health to Revitalize Japan: Hopes for the New Administration (December 1, 2025)
- [Announcement] HGPI Joins Global Green and Healthy Hospitals (August 1, 2023)
- [Research Report] Survey of Japanese Nursing Professionals Regarding Climate Change and Health (Final Version) (November 14, 2024)
Featured Posts
-
2025-12-09
[Event Report] Special Seminar “Rising to New Challenges in Health Sciences for Future Society: Novel Developments in the Field of Epilepsy in Japan and Globally” Belgium Pavilion Special Seminar, World Expo 2025 Osaka, Kansai (September 18, 2025)
![[Event Report] Special Seminar “Rising to New Challenges in Health Sciences for Future Society: Novel Developments in the Field of Epilepsy in Japan and Globally” Belgium Pavilion Special Seminar, World Expo 2025 Osaka, Kansai (September 18, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20250805_mental-health-expo-eyechatch.png)
-
2025-12-11
[Event Report] Core Components of Universal Health Coverage (UHC): Achieving “Healthcare Without Financial Hardship” in Asia-Pacific and Japan (December 5, 2025)
![[Event Report] Core Components of Universal Health Coverage (UHC): Achieving “Healthcare Without Financial Hardship” in Asia-Pacific and Japan (December 5, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20251210_Core-Components-of-Universal-Health-CoverageUHC-top.jpg)
-
2025-12-12
[Registration Open] Meaningful Involvement Promotion Project Urgent Symposium “The New Takaichi Administration and Central Social Insurance Medical Council Reform – Ensuring Patients’ Voices are Heard” (January 22, 2026)
![[Registration Open] Meaningful Involvement Promotion Project Urgent Symposium “The New Takaichi Administration and Central Social Insurance Medical Council Reform – Ensuring Patients’ Voices are Heard” (January 22, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20251208_urgent-symposium-1.png)
-
2025-12-12
[Registration Open] (Webinar) The 140th HGPI Seminar “Early Detection to Reduce COPD Disease Burden: Connecting Clinical Frontiers with Health Policy” (January 27, 2026)
![[Registration Open] (Webinar) The 140th HGPI Seminar “Early Detection to Reduce COPD Disease Burden: Connecting Clinical Frontiers with Health Policy” (January 27, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs140-top.png)
-
2025-12-16
[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)
![[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/eyecatch_Policy-Dialogue_Discussion-Points_20251128.jpg)